PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
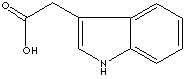
c12c([nH]cc1CC(O)=O)cccc2
CLASSIFICATION
Plant growth regulator
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
SOLVENT SOLUBILITY
5
REFRACTIVE INDEX
Stable under ordinary conditions (light sensitive)
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Indole-3-acetic acid
Google Scholar Search - Indole-3-acetic acid
Drug Information Portal (U.S. National Library of Medicine) - Indole-3-acetic acid
PubChem Compound Summary - Indole-3-acetic acid
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Indole-3-acetic acid
http://www.ebi.ac.uk/ - Indole-3-acetic acid
http://www.ncbi.nlm.nih.gov/ - Indole-3-acetic acid
Local: Auxin is one of five (or more) major plant hormones (Auxin, Cytokinins, Gibberellins, Ethylene and Abscisic acid) which affect numerous plant growth processes functions including cell division and elongation, autumnal loss of leaves, and the formation of buds, roots, flowers, and fruit. Auxin action is inhibited by light which is an important role of the growth of stems toward light (phototropism), against the force of gravity (geotropism) and positively hydrotropic (moisture-seeking). The cells exposed to light don't grow as quickly as those on the shaded side, and thus the plant grows toward the light source. Auxins usually have a ring system with at least one double bond and attached by a side-chain that terminates in a carboxyl group. Indole acetic acid is the exact structure of Auxin activity. Parent compounds of auxin action are;
- Auxins
- 4-Chlorophenoxyacetic acid (CAS RN: 122-88-3)
- (2,4-Dichlorophenoxy)acetic acid (CAS RN: 94-75-7)
- 4-(2,4-Dichlorophenoxy)butyric acid (CAS RN: 94-82-6)
- Tris[2-(2,4-Dichlorophenoxy)ethyl] phosphite (CAS RN: 94-84-8)
- 2-(2,4-Dichlorophenoxy)propanoic acid (CAS RN: 120-36-5)
- 2-(2,4,5-trichlorophenoxy)propanoic acid (CAS RN: 93-72-1)
- Indole-3-acetic acid (CAS RN: 87-51-4)
- Indole-3-butyric acid (CAS RN: 133-32-4)
- 1-Naphthaleneacetamide (CAS RN: 86-86-2)
- 1-Naphthaleneacetic acid (CAS RN: 86-87-3)
- 1-Naphthol (CAS RN: 90-15-3)
- Naphthoxy acetic acid (CAS RN: 120-23-0)
- Naphthenic acid, inorganic salts (potassium, sodium)
- (2,4,5-Trichlorophenoxy) Acetic acid (CAS RN: 93-76-5)
- Antiauxins
- Clofibric acid (CAS RN: 882-09-7)
- 2,3,5-Triiodobenzoic acid (CAS RN: 88-82-4)
Cytokinin is a N6-substituted adenines acting as phytohormones such as kinetin, zeatin, 6-isopentenyladenine, benzyl adenine. The principal functions are stimulate cell division in concert with auxin (cytokinesis) and influence the pathway of tissue differentiation (organogenesis). 6-Benzylaminopurine is the first generation synthetic cytokinin which elicits plant growth and development responses setting blossoms and stimulating fruit richness by stimulating cell division. Active cytokinin ingredients include:
- Adenine (CAS RN: 73-24-5)
- Adenine Hemisulfate salt (CAS RN: 321-30-2)
- 6-Benzylaminopurine (CAS RN: 1214-39-7(base), 162714-86-5(HCl)
- N-Benzyl-9-(2-tetrahydropyranyl)adenine (CAS RN: 2312-73-4)
- N-(2-Chloro-4-pyridyl)-N'-phenylurea (CAS RN: 68157-60-8)
- 6-(gamma,gamma-Dimethylallylamino)purine (CAS RN: 2365-40-4)
- 1,3-Diphenylurea (CAS RN: 102-07-8)
- Kinetin (CAS RN: 525-79-1 (base), 177966-68-6 (HCl)
- 1-Phenyl-3-(1,2,3-thiadiazol-5-yl) Urea (CAS RN: 51707-55-2)
- Zeatin (CAS RN: 13114-27-7)
- trans-Zeatin (CAS RN: 1637-39-4 (base), 6025-81-6 (HCl))
- trans-Zeatin riboside (CAS RN: 6025-53-2)
Other Plant Growth Regulators include:
- Abscisic acid (CAS RN: 21293-29-8)
- Ancymidol (CAS RN: 12771-68-5)
- Chlorocholine chloride (CAS RN: 999-81-5)
- Daminozide (CAS RN: 1596-84-5)
- 3,6-Dichloro-o-anisic acid (CAS RN: 1918-00-9)
- Gibberellic acid (CAS RN: 77-06-5)
- Gibberellic acid Potassium salt (CAS RN: 125-67-7)
- Gibberellin A4 (CAS RN: 468-44-0 ) and other gibberellins (more than 110 gibberellins are known)
- Glyphosate (CAS RN: 1071-83-6)
- Jasmonic acid (CAS RN: 3572-66-5)
- 1,3,5-Trihydroxybenzene (CAS RN: 108-73-6)
APPEARANCE
ACTIVE CONTENT
98.0% min
HAZARD OVERVIEW
May cause eye, skin, and respiratory tract irritation.
GHS
PICTOGRAMS

HAZARD STATEMENTS
H315-H319-H335
P STATEMENTS
P261-P280-P302+ P352-5-P305 + P351 + P338
![]()
RISK PHRASES
36/37/38
SAFETY PHRASES
26-37/39